Bien-Air Select Partner
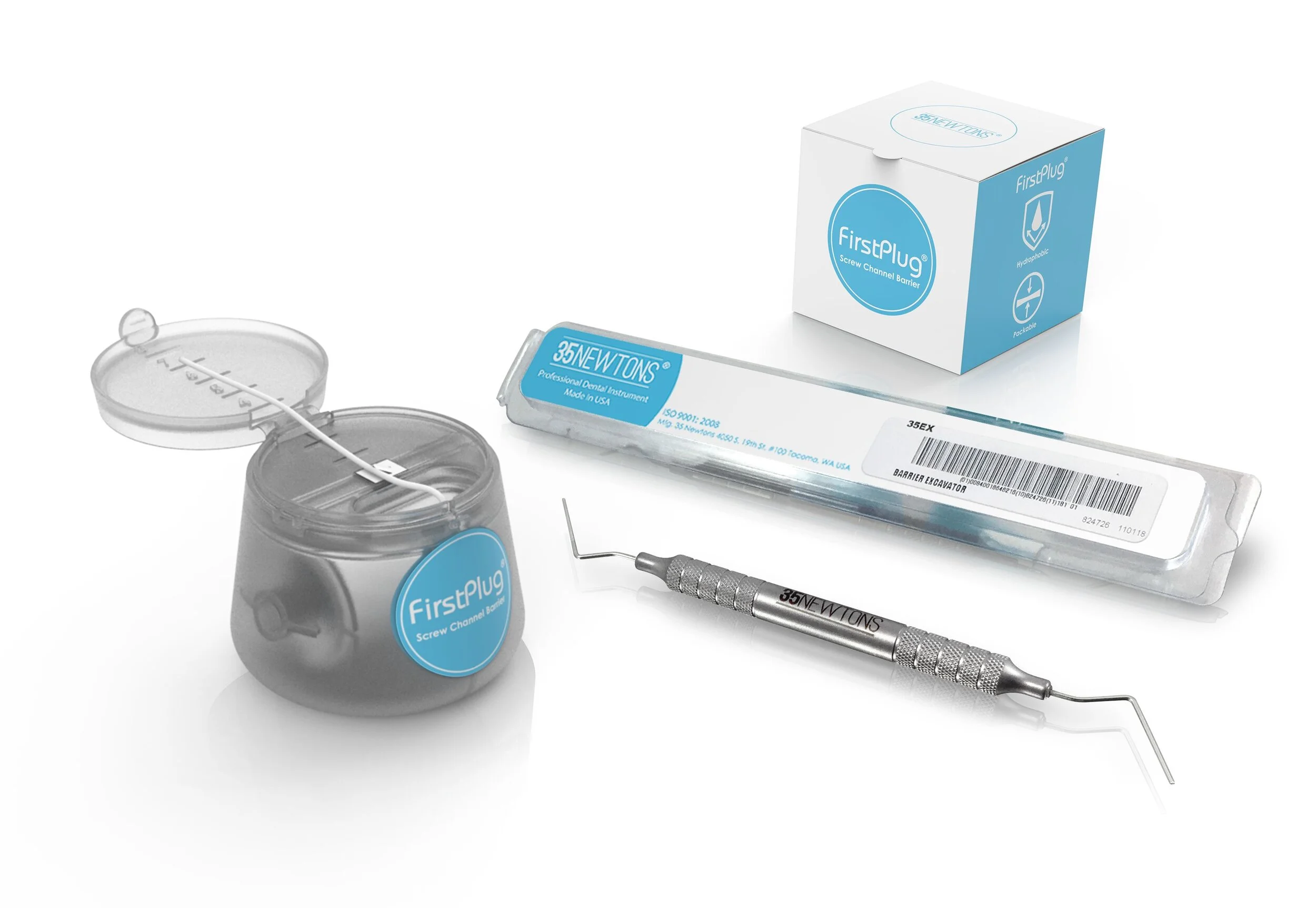
FirstPlug
FirstPlug™ from 35 Newtons, brings a medical grade solution to the clinically sensitive task of filling access channels in screw retained restorations. FirstPlug is made from medical grade PTFE (polytetrafluoroethylene), and has been designed with handling characteristics that make it ideal for packing and sealing screw access chambers. The accompanying FirstPlug instrument set, which includes a wide diameter barrier plugger and barrier excavator, has been optimized for efficient placement and removal of the FirstPlug material.
Sign up for free trial samples of FirstPlug and receive complementary FirstPlug instrument set worth over $100.
The launch of FirstPlug™ from 35 Newtons, brings a much needed upgrade to the clinically sensitive task of filling access channels in screw retained restorations. The patented system consists of a medical grade PTFE barrier material and an instrument set optimized for packing or filling retention screw channels during restorative procedures.
Material selection for covering implant abutment screws is important to limit leakage which has been shown to negatively impact peri-implant tissues. “None of the common materials and techniques in use today were developed specifically for this procedure and all have their drawbacks,” said Dr. Jim Janakievski, a Tacoma area periodontist, and co-founder of 35 Newtons. For example, cotton pellets provide an ideal substrate for pathogenic oral flora to flourish, while gutta percha can be difficult to remove. “The ideal barrier material provides a good seal to limit leakage, is pliable, biologically inert, and easily retrievable,” said Dr. Alex Shor, prosthodontist and 35 Newtons co-founder.
FirstPlug is made from PTFE (polytetrafluoroethylene), which at a basic level is similar to the material used to make industrial plumber’s tape. “Many dentists are creative problem solvers and lacking suitable alternatives some have undoubtedly resorted to the local hardware store for an answer,” said Dr. Janakievski. “PTFE is the right material, but we feel strongly that implants and other sensitive dental procedures call for a medical grade solution.”
Every detail the FirstPlug system has been engineered to fit the clinical application. The large diameter cylindrically shaped cord seats easily in the screw channel without rolling or twisting. The medical grade PTFE has the right compression characteristics for packing and sealing the screw access chamber, as well as sufficient stiffness to support the overlying restorative materials. The accompanying instrument set includes a wide diameter barrier plugger for efficient packing of the PTFE cord, as well as a barrier excavator for easy removal when uncovering the screw to remove temporaries or replace a restoration.
In addition to the optimized physical characteristics of the medical grade PTFE material itself, FirstPlug is manufactured, assembled, and packaged under strict medical device protocols and quality systems. “Of course, FirstPlug users appreciate the added convenience, efficiency, and clinical benefits,” said Dr Shor. “But first and foremost, we hear from our dentists that sealing implant access channels with a medical grade material, made in medical class conditions offers invaluable peace of mind.”